High-Pressure Volumetric Gas Sorption Analyzer:
iSorb
- One-station gas sorption analyzer
- High-pressure measurements up to 100 bar pressure
The iSorb HP gas sorption analyzers are perfect for evaluating materials in gas storage, gas separation, or emission control applications. With high-precision transducers, precise manifold temperature control, and a built-in library of advanced equations of state, the iSorb HP series gives you access to high-quality gas adsorption and kinetic data to a maximum of either 100 bar or 200 bar absolute. Optional temperature control accessories allow measurements over a temperature range of 75 K to 773 K.
Key features
Robust manifold design ensures results unaffected by temperature and ambient conditions
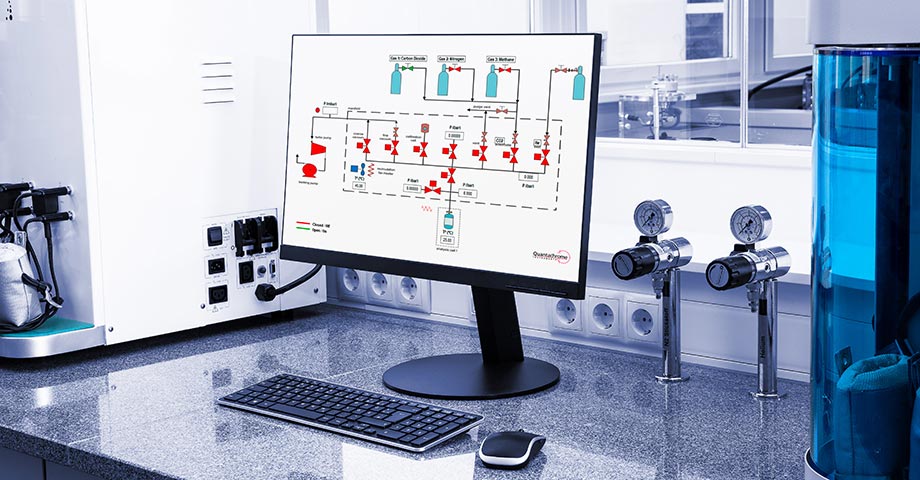
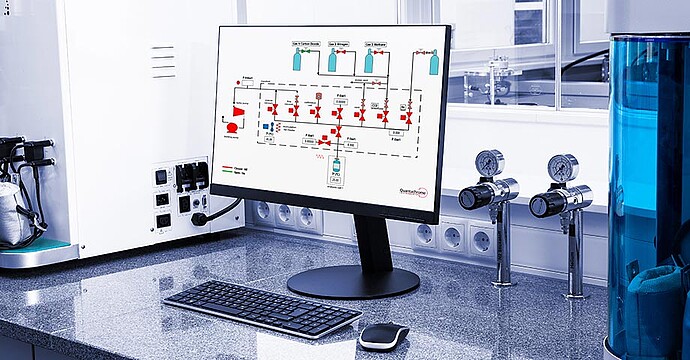
High-pressure experiments require well-controlled temperature for accurate and precise measurements. Therefore, the manifold of the iSorb HP is thermostatically heated above room temperature for better stability and isolation from environmental changes. Microprocessor-based digital pressure transducers provide temperature-compensated pressure readings. The software monitors the stability of the manifold temperature and gas pressure before dosing into the sample cell; readings taken at stable temperatures provide for the most precise and accurate measurements. Metal VCR seals and pneumatic valves ensure leak-tight operation.

Highly flexible experimental conditions
iSorb HP features a variety of high pressure sample cells that accommodate a wide range of sample types: powders, granules, films, and monoliths. Sample temperature is controlled by several accessories from cryogenic (75 K) to elevated temperatures (773 K). The low-volume micro cell enables you to determine the performance of your materials even when sample amount is limited. The reduced void space and excellent temperature control help eliminate the need to perform blank subtraction runs. The compact pressure booster produces a 4:1 compression result maintaining 200 bar with as little as 55 bar left in the gas cylinder to save you money and resources.

Peace of mind with built-in safety
Working at higher pressures with hazardous gases means safety is of utmost concern. With the iSorb HP, sample cell pressure is constantly monitored, even in the event of a power outage; during a power outage the instrument will automatically close all valves. Its “Safe State” routine returns the manifold and sample cell to ambient pressure with helium enabling easy sample removal. The intelligent software has an automatic leak detection algorithm that can differentiate between adsorption and a leak. If a leak is detected, the instrument will stop the analysis and return to a safe state, and can trigger an external alarm. An automated supply-line cleaning routine prevents mixing of two incompatible gases.

Insights into sample kinetics, heat adsorption at different temperatures, and more
Surface excess isotherms are easily obtained with the iSorb HP with two built-in methods: classical target pressure mode or faster pressure dosing. You can also explore the sample kinetics of each data point collected and calculate the heat of adsorption from multiple isotherms collected at different analysis temperatures. Absolute adsorbed amount isotherm and storage capacity calculations will aid R&D in finding the next novel material for gas storage capabilities.

Permanent control of instrument status and health
The valve management system records each cycle of the valves to help you plan your maintenance schedule. The exportable graphical log file records all pressure and temperature readings and valve operations along with animated graphics for rapid diagnostics and complete analysis playback.
Technical specifications
| Performance/physical | iSorb HP1 | iSorb HP2 |
|---|---|---|
| Analysis stations | 1 | 2 |
| Gas inputs | 2 (optional 4) | |
| Maximum pressure data | 100 bar or 200 bar absolute | |
| Minimum pressure data | 0.0005 bar | |
| Low Pressure (LP) transducers | 2 (1 bar) | 3 (1 bar) |
| High Pressure (HP) transducers | 2 | 3 |
| Total transducer count | 4 | 6 |
| Transducer accuracy | <±0.05 % f.s | |
| Vacuum pump | internal | |
| Turbo pump option | yes | |
| Degas type | automatic | |
| Degas ports | in-situ | |
| Max. degas temp | 500 °C | |
| Thermostatted bath option | yes | |
| Cryo option | yes | |
| Booster option | yes | |
| Thermostatted manifold | yes | |
| Gas inputs/vents location | Side, for easy access | |
| Dimensions (W x H x D) | 85 cm x 100 cm x 50.5 cm (33.5 in x 39.25 in x 19.5 in) | |
| Weight | 150 kg (330 lbs) | 150 kg (330 lbs) |
Anton Paar Certified Service
- More than 350 manufacturer-certified technical experts worldwide
- Qualified support in your local language
- Protection for your investment throughout its lifecycle
- 3-year warranty
Documents
-
High Pressure CO2 Sorption on Porous Carbons Application Reports
-
High Pressure CO2 and CH4 Sorption on Zeolites Application Reports
-
Material Brief: Metal Hydride - Hydrogen Absorption - iSorb Application Reports
-
Porous Carbon – Hydrocarbon Adsorption – iSorb HP Application Reports
-
Advanced sorption suite Brochures
-
NEW HORIZONS IN PARTICLE ANALYSIS Brochures
-
iSorb HP Brochure Brochures